
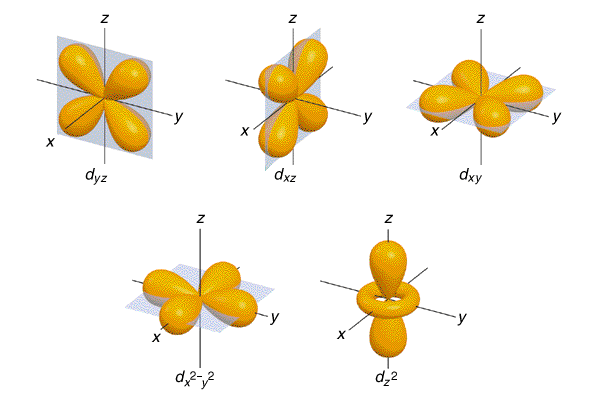
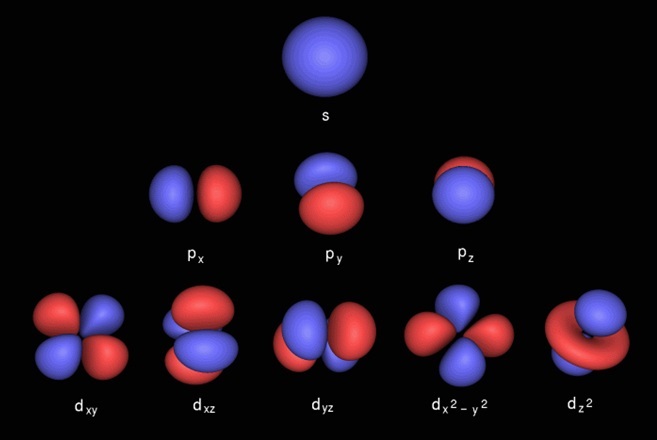
After the 3D orbital is full, the sequence continues to 4P. After the addition of the 4S electrons and before the addition of the 4P electrons, the sequence reverts to the third energy level to insert electrons in a 3D orbital. We encounter 4S with Potassium (K) and Calcium (Ca), but at Scandium (Sc) the pattern changes. N=3 is constructed the same way as the second - with 3S filling before 3P. Sodium (Na) is the first element to use the third energy level. The 2S orbital is shaped like a sphere the three 2P's are each shaped like a dumbell, intersecting at their centers at right angles to each other. The 2S is slightly lower in energy than the 2P's and thus fills first the 2P's are all equal to each other in energy. Each of the four subshells can contain two electrons, so in total the n=2 shell has room for eight electrons. The n=2 shell is constructed of two orbitals: 2S and 2P, and the 2P is further broken down into three subshells: 2Px, 2Py, and 2Pz. Berillium (Be), number 4 in the Periodic Table, fills 2S with its two electrons.īoron, though, begins to make use of the n=2 shell's more complex structure. Lithium has two electrons in orbital 1S, and one electron in an n=2 orbital: 2S. Its two electrons fill the 1S orbital.īeginning with Lithium (Li), with atomic number 3, there is not room in the n=1 energy level for all the electrons, so the n=2 level begins to fill. Its single electron travels around the proton in the first energy level, n=1 (where n is the integer rank number of the level and goes from 1 to infinity.) The n=1 energy level consists of a single 1S orbital, which is shaped like a sphere, centered on the nucleus (the nomenclature "S" is an historic artifact and is not significant.) Element with atomic number 2, Helium (He), contains two protons and two neutrons in its nucleus. The first element, Hydrogen (H), has an atomic number of Z=1. This value is called the atomic number of the element. This can get confusing!Įlements are classified by the number of protons in their nucleus - a number usually denoted by the letter Z. Now, before you read any further, you might want to have the PERIODIC TABLE OF THE ELEMENTS handy, to follow along. Electrons "fill up" orbitals from the lowest energy up - that is, the second orbital does not contain an electron unless the first orbital is already full. A subshell has a place for up to two electrons. Each orbital contains one or more subshells. Here, we will only describe ground state electron configurations.Įach energy level, or shell, contains one or more orbitals. In this case, we say that the atom is in an excited state. Through energy transfers during collisions with other particles or with light, it is possible for electrons to move up to orbitals of higher energy. The farther an electron orbits from the nucleus, the higher the energy associated with it. The electrons orbit only in certain "allowed" regions around the nucleus. THE CLASSICAL MODEL OF ELECTRON ORBITAL CONFIGURATION This is a simplified scenario in which we regard the electrons as discrete objects orbiting at known, fixed distances from the nucleus. As an introduction, it's useful to present the Classical Model of atomic structure.
The true nature of the electrons' movement around the nucleus is complex. The positive and negative charges cancel, and thus the net charge of the atom is zero. An atom in its neutral state always has the same number of protons as electrons. Electrons are bound to the nucleus by their electromagnetic attraction to the positively-charged protons. The electron carries a negative charge: -1e.
The neutron has no charge (it is electrically neutral.) For convenience, the charges of subatomic particles are usually described as multiples of the value e, the charge of one electron, called the elementary charge.) The proton has an electric charge of +1e, or 1.602 x 10 -19 Coulombs (a Coulomb is a unit of electric charge. For the purposes of chemistry, we are interested in three of these particles: the proton, the neutron, and the electron.Īn atom consists of a central nucleus of protons and neutrons, and electrons that orbit the nucleus.
Over the past two centuries, experimentalists have identified numerous particles that comprise an atom. It is not indivisible, though, as the Greek origin of its name indicates. As a result they interact and their energies change, one is pushed up and one is pushed down.The atom is the building block of matter. For period 2 diatomics, this occurs for $\ce$ orbitals are such that they can constructively and destructively overlap. According to molecular orbital theory s and p orbitals can mix if they are close enough in energy to each other.


 0 kommentar(er)
0 kommentar(er)
